"Tomorrow's cure lies not in a universal pill, but within our own genetic code." - Futurist Jim Carroll

(Futurist Jim Carroll is writing a series on 30 Megatrends, which he first outlined in his book Dancing in the Rain: How Bold Leaders Grow Stronger in Stormy Times. The trends were shared in the book as a way of demonstrating that, despite any period of economic volatility, there is always long-term opportunity to be found. The book is now in print - learn more at dancing.jimcarroll.com)
The one-size-fits-all era in medicine is ending. Treatments, nutrition, and wellness approaches tailored to individual genetic profiles are becoming the standard, not the exception. As with every post in this series, there's a PDF here that goes into more depth on this trend.

There's a little bit of duplication with some of the material covered in previous megatrends with this one, but it's important ot note that this trend is powerful enough on its own to deserve its own post.
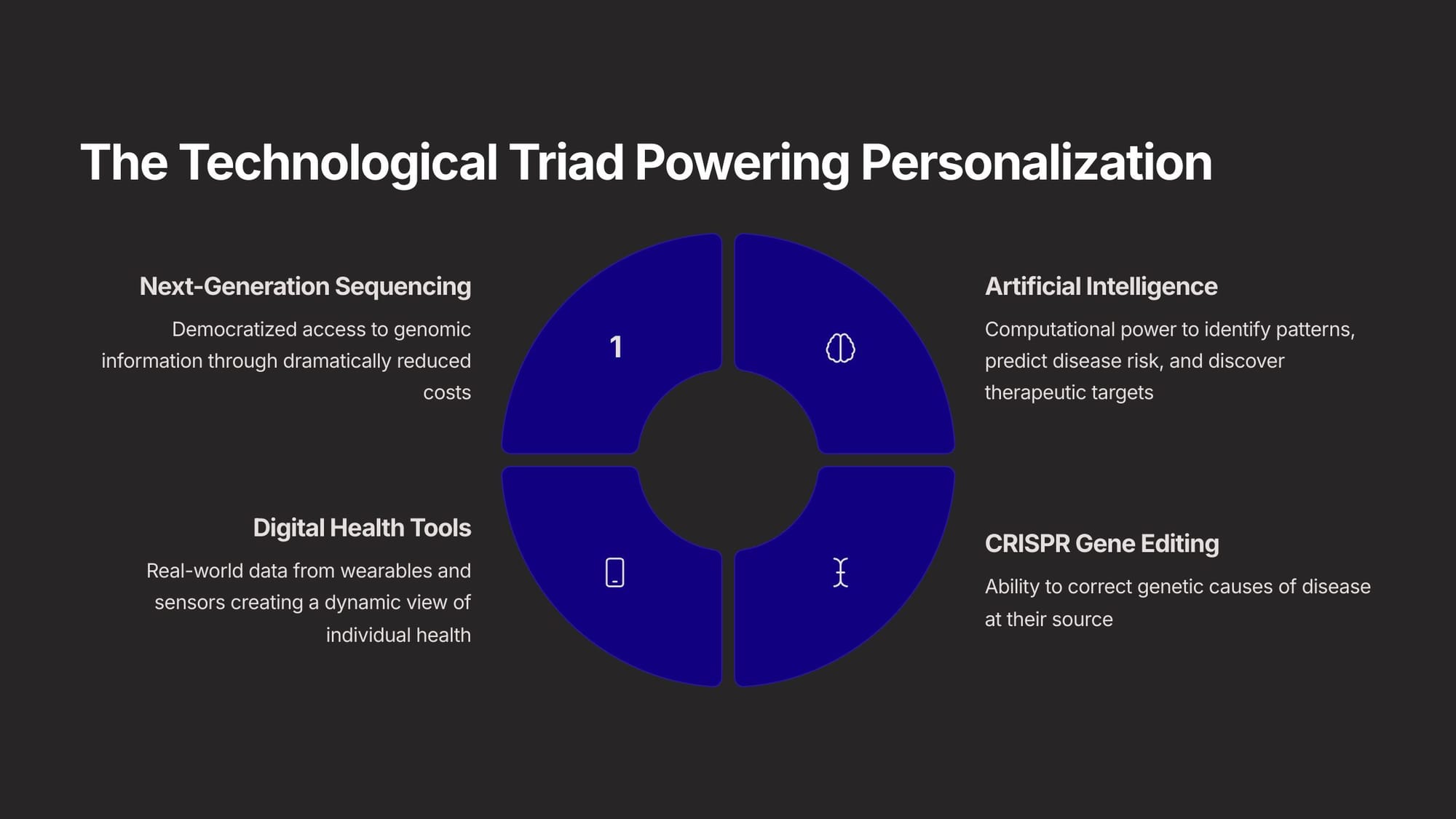
What's it all about? We are combining what we might call 'next-generation genomic sequencing', AI, and CRISPR gene editing, to come up with a new health paradigm that involves 'fixing people before they are sick rather than after.' Why these 3 trends? The cost for genomic sequencing is collapsing, AI is accelerating the ability to do so, and gene editing allows us to correct the genetic causes of disease at the source.
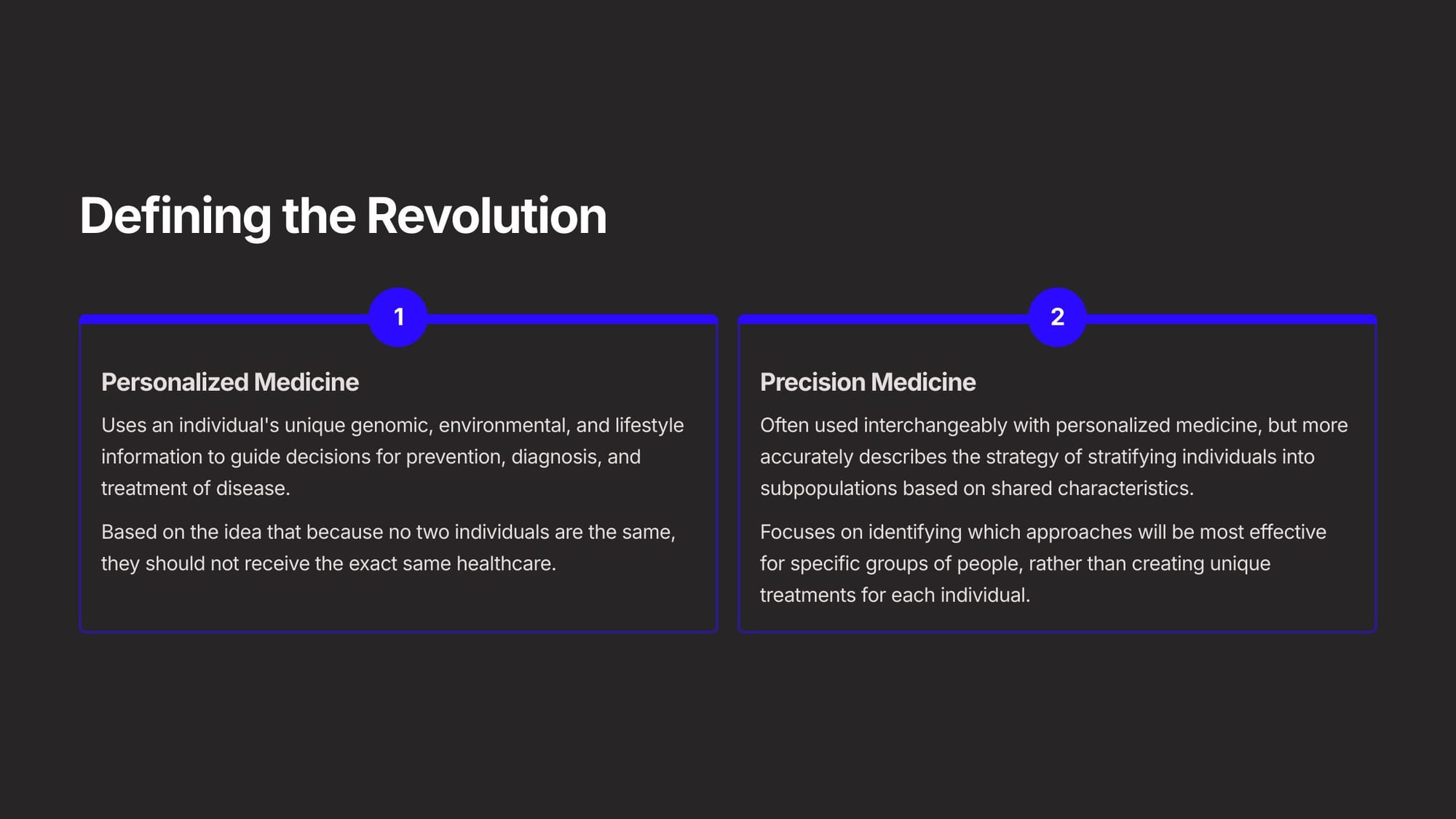
Before going further, it's important to define the revolution by understanding two key terms.
The first is Personalized Medicine, which uses an individual's unique genomic, environmental, and lifestyle information to guide decisions for prevention, diagnosis, and treatment of disease. It's based on the idea that because no two individuals are the same, they should not receive the same healthcare.
The second term is Precision Medicine. While often used interchangeably, it more accurately describes the strategy of stratifying individuals into small populations based on shared characteristics. It focuses on identifying which healthcare and pharmaceutical approaches will be most effective for those small, specific groups of people, rather than creating unique treatments for each individual.
This transformative shift in healthcare is further characterized by the P4 Medicine Framework. This framework emphasizes that medicine is becoming increasingly Personalized, where risks and treatment responses are unique to each individual's profile. It is also becoming Predictive, aiming to forecast the likelihood of developing conditions for proactive management. Furthermore, it is Preventive, offering targeted measures to reduce disease risk, and crucially, Participatory, requiring and empowering the active involvement of patients in their own healthcare journey. This framework highlights a move towards a more collaborative partnership between healthcare providers and patients.

The scientific bedrock of this personalized medicine is genomics, catalyzed by the Human Genome Project (HGP). Completed in the early 2000s, the HGP provided the first comprehensive sequence of the human genome—the complete set of DNA instructions for building and maintaining a human being. This monumental achievement mapped approximately 3 billion base pairs and 20,000-30,000 genes, launching the modern era of genomics and making personalized medicine a tangible possibility. That was a huge development in the evolution of this megatrend.

That came about as we saw the emergence of several transformative technologies - wearables, AI, and gene editing. These are the engines of the personalized era. A convergence of capabilities—allowing us to read, write, and interpret the code of life with unprecedented efficiency—has created a powerful engine driving the entire field forward.
One of these key capabilities is "writing" the code. If Next-Generation Sequencing (NGS) gave us the ability to read the code of life, CRISPR-Cas9 gives us the ability to write it, or 'edit' it. This allows scientists can introduce specific changes, opening up revolutionary possibilities for directly correcting underlying genetic mutations that cause many diseases and conditions. This is a pretty profound opportunity.

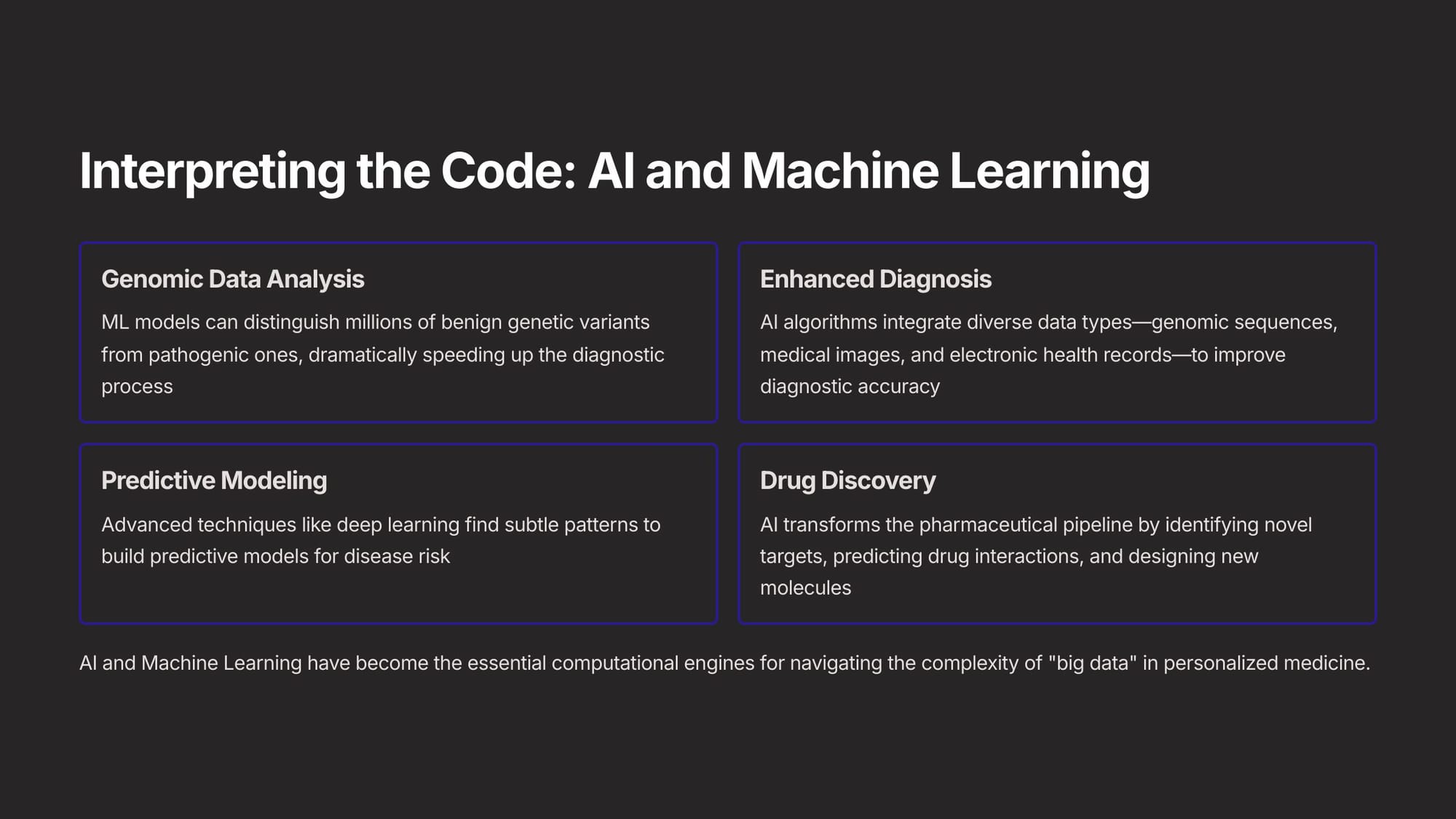
The next essential capability is "interpreting" the DNA code, a task managed by AI. These have become the essential computational engines for navigating the complexity of "big data" in personalized medicine. Essentially, AI now allows us to perform very detailed genomic data analysis to distinguish problem genes from good ones. This allows us to do two things - identify disease risk in particular people, and engineer very specific drugs for specific people.
The final technological pillar extends beyond the laboratory into daily life, allowing us to "live" the code - wearables!. Digital health technologies and wearables provide a continuous, real-time stream of data about an individual's physiology and behavior, including physical activity, sleep quality, heart rate, stress levels, and blood glucose. This creates a dynamic, high-resolution "digital map" that moves personalized medicine beyond a static snapshot to a real-time view of health.

In other words, we know what people might become sick with, treat them before it becomes more serious, and then monitor them in time over time.
While we've been talking about this capability for years, this theoretical promise of personalized medicine is rapidly translating from theory to therapy. It is making a tangible clinical impact across a growing number of medical specialties. From optimizing drug prescriptions to transforming cancer treatment and offering new hope for rare diseases, the application of genomic and molecular insights is fundamentally changing how clinicians prevent, diagnose, and manage illness.

A prime example of this is Pharmacogenomics (PGx), the study of how an individual's genetic makeup influences their response to medications. Instead of the traditional one-size-fits-all approach, genetic testing can identify variants affecting drug metabolism. This allows for personalized dosing to determine the optimal drug and dose based on a person's unique genetic profile. As studies indicate that more than 98% of people may carry at least one such variant, PGx is one of the most broadly applicable pillars of personalized medicine.

We needn't stop there - why not use the same genetic insight and wearable monitoring to provide specific, healthier food options particular to a person's specific DNA? After all, my glycemic index for an apple might be different than yours. This leads us into the idea of healthier eating based on specific genetic profiles - a very profound change. This is called 'nutrigenomics,' and it too is a rapidly growing field that studies how nutrients and dietary compounds interact with an individual's genes to influence their health. This field aims to tailor dietary, supplement, and lifestyle recommendations based on a person's unique characteristics, including their health goals, medical conditions, activity level, genetic predispositions, and even their microbiome composition and blood biomarkers.

While its potential is immense, the transition to personalized medicine is not merely a technological upgrade but a disruptive force that challenges the foundational structures of healthcare delivery, research, and regulation.

This is a tremendously complicated megatrend with HUGE implications, and realizing its potential on a global scale requires confronting a formidable set of interconnected challenges!
Even so, over the long term, it's one of the biggest megatrend opportunities of our time.
Futurist Jim Carroll first covered the personal and precision medical trend in his 1997 book, Good Health Online.

